ESTROGEN GUY
VET
- Joined
- Aug 3, 2018
- Messages
- 2,366
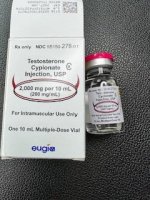
Eugia Pharma receives USFDA Approval for Testosterone Cypionate Injection USP 1,000 mg/10 mL (100 mg/mL) and 2,000 mg/10 mL (200 mg/mL) in Multi-Dose Vial and 200 mg/mL in Single-Dose Vial
PUBLISHED : OCTOBER 24, 2023Ref: Aurobindo Pharma
Aurobindo Pharma Limited is pleased to announce that its wholly owned subsidiary company, Eugia Pharma Specialities Limited, has received final approval from the US Food & Drug Administration (USFDA) to manufacture and market Testosterone Cypionate Injection USP 1,000 mg/10 mL (100 mg/mL) and 2,000 mg/10 mL (200 mg/mL) in Multi-Dose Vial and 200 mg/mL in Single-Dose Vial, which is bioequivalent and therapeutically equivalent to the reference listed drug (RLD), Depo-Testosterone Injection, 100 mg/mL and 200 mg/mL of Pfizer Inc. The product is expected to be launched in November 2023. The approved product has an estimated market size of US$ 226.8 million for the twelve months ending August 2023, according to IQVIA.
This is the 169th ANDA approval (including 9 tentative approvals received) out of Eugia Pharma Speciality Group (EPSG) facilities, manufacturing both oral and sterile specialty products.
Testosterone Cypionate Injection USP is indicated replacement therapy in the male in conditions associated with symptoms of deficiency or absence of endogenous testosterone, Primary hypogonadism (congenital or acquired) and Hypogonadotropic hypogonadism (congenital or acquired).